
Difference between ind and nda application - Brainly. What is the difference between NDA I and NDA II? When is an IND required?
The application is submitted to the United States Food and Drug Administration (US FDA) for obtaining exemption to ship the product to investigators across the state. In order to obtain this exemption, the company must provide all the necessary information through the IND. There are two types of INDs: 1. Commercial – For companies planning to obtain marketing approval for a new drug 2. See full list on freyrsolutions. The information of the IND can be categorized into three broad areas: 1. Animal Pharmacology and Toxicology Studies – This section contains all the information deemed necessary to ensure that the drug is safe for initial human testing. It also includes any previous history of use of the drug on humans.
Manufacturing Information – This section contains information that ensures the capability of the manufacturing unit for producing adequate batches of the drug. Clinical Protocols and Investigator Information – This section contains information to identify if the initial tests could pose a risk to human. For any drug to obtain approval for sales and marketing in the U. It is a comprehensive document with sections that provides data on animal and human studies, pharmacology of the drug, toxicology, and dosage, and contains information about the drug’s manufacturing process.
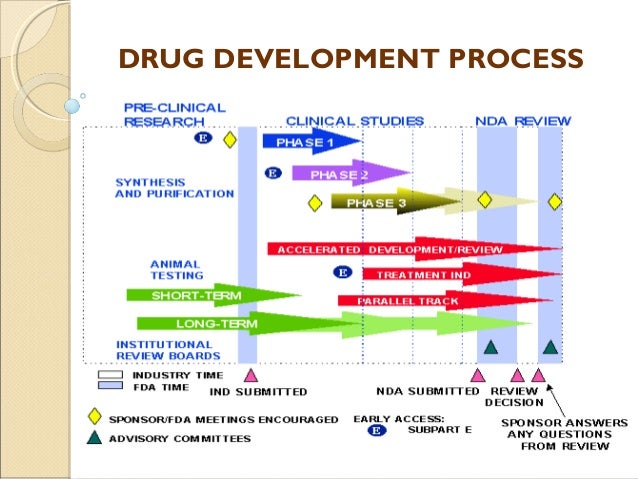
New Drug Application (NDA). The purpose of an NDA is to provide the FDA reviewer adequate data to ensure the safety and efficacy of the drug, labeling, and manufacturing process. Once submitte the FDA takes days to decide whether to review the application or reject it, due to missing information.
Once the FDA has reviewed the NDA, it issues one of the below mentioned three action letters: 1. Approval Letter – Indicates that the drug is approved 2. Approvable Letter – Indicates that the drug will be approved eventually, but requires rectification due to a few inadequacies such as labeling changes 3. An NDA is an application to permit the sale and marketing of a new drug in the United States. A traditional NDA consists of data and information about the drug as gained from both nonclinical and clinical studies, as well as a summary of formulation development and manufacturing processes, and proposed labeling information to be included in the drug’s packaging. In general, an NDA should contain enough data for the FDA to determine if the drug is safe and effective for its proposed use, if the benefits of taking the drug outweigh the risks, and if the drug product is manufactured in a way that preserves its identity, strength, quality, and purity. A BLA is a request to introduce, or deliver for introduction, a biological product into interstate commerce.
Like an NDA, a BLA should include all information about the biological product that was gained over the developmen. Because biological products are typically derived from living systems, their large, complex structures are often difficult to characterize. This is a key distinction from traditional drug molecules, which are chemically synthesized and structurally both simpler and smaller in size. The manufacturing process for biological products is also more complicate due to genetic variability in the source material.
Because of this, it is critical that BLAs contain a thorough description of product development and relevant manufactu. While BLAs and NDAs serve the same purpose of gaining approval to market a drug in the United States, they differ slightly in terms of their application content and submission requirements. Regarding approval criteria, NDAs must fulfill three conditions: 1. The drug is safe and effective for the proposed use and that the benefits outweigh the risks 2. The labeling is appropriate and contains all necessary information about the drug 3. Due to the complexities of manufacturing biological products, a pre-license inspection of the facility is generally required before a BLA is approved. Pre-approval inspection. While all conventional drug products (i.e., small molecules) are regulated by CDER, biological products can be regulated by either CDER or CBER, depending on the product’s classification as discussed above.
These product categories include monoclonal antibodies for in vivo use, most proteins for therapeutic use (e.g., cytokines, enzymes, and other novel proteins except those assigned to CBER, such as vaccines and blood products), immunomodulators, and growth factors. Regardless of the category, NDAs for all drug products fall under the jurisdiction of CDER. Like an NDA , a BLA is submitted to the FDA in order to market a new drug in the US.
Additionally, many of the same regulations apply to NDAs and BLAs, including labeling and advertising rules, accelerated approval pathways, pediatric study requirements, and PDUFA fees. This includes initial filing of an INDand subsequent maintenance of the IND throughout the drug development program until the marketing application is submitted. The structure and contents of the IND do not differ between drugs and biological products. Along with introducing an abbreviated approval pathway for highly similar biological products (i.e., biosimilars), this act mandated that moving forwar all biological products must be submitted for marketing approval through a BLA, and not an NDA. While they are both submitted to gain FDA drug approval, they differ in terms of product categories, approval criteria, and certain regulations.
NDAs and BLAs are the two types of applications that are submitted in order to market a new drug in the United States. Nuventra consultants are widely experienced in the preparation and submission of both BLAs and NDAs for numerous drugs and indications. If you have questions about preparing your marketing application or need assistance with its development, Nuventra is here to help. Contact us today to see how we can support you on your pathway to FDA approval.
Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement. The IND is the means through which the sponsor technically obtains this exemption from the FDA.
The following resources include the legal requirements of an IND application, assistance from CDER to help you meet those requirements, and internal IND review principles, policies and procedures. They also establish policies intended to achieve. Register and Subscribe now to work with legal documents online. Instant Downloa Mail Paper Copy or Hard Copy Delivery, Start and Order Now! Mutual NDA vs NDA are two types of NDAs, or non-disclosure agreements, in the U. They are usually used to protect certain confidential information from wrong exposure, theft, or misuse.
The IND follows the CTD structure developed by ICH and requires very detailed product and development data such as information of manufacture, data from nonclinical. The IND application is submitted by the company or research group responsible for. An abbreviated new drug application (ANDA) contains data that, when submitted to the FDA, provides for the review and ultimate approval of a generic drug product.

Instantly F ind and Download Legal Forms Drafted by Attorneys for Your State.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.