FDA Drug Approval Process - Drugs. What is the approval process? Electronic submissions should be considered whenever. Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines.

Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement. The IND is the means through which the sponsor technically obtains this exemption from the FDA. See full list on fda.
The following resources include the legal requirements of an IND application, assistance from CDER to help you meet those requirements, and internal IND review principles, policies and procedures. The review divisions are organized generally along therapeutic class and can each be contacted using the designated Pre-IND Consultation List (PDF - 19KB). They also establish policies intended to achieve. Expanded Access to Experimental Biologics Expanded access, sometimes called compassionate use, is the use outside of a. Other accommodations for usage prior to approval include treatment IND and parallel tracking. Once phase III is complete, the manufacturer files an NDA.

How to Meet IND Filing Requirements. An investigational new drug application (IND) outlines what the sponsor of a new drug proposes for human testing in clinical trials. When a drug reaches this. Phases of Human Testing for Investigational Drugs. New Drug Application (NDA).
An approval process is the method an organization uses to approve anything from documents , invoices , budgets , and purchase orders , to a new process that a company wants to institute. Implementing an approval process can standardize an organization’s internal processes, and also save time by creating a dependable, repeatable system. Like general drug approval process, FDA’s new drug approval process is also accomplished in two phases: clinical trials (CT) and new drug application (NDA) approval. FDA approval process begins only after submission of investigational new drug (IND) application. Submission on this application is based on the of initial testing of the drug.
The application will contain information on the drug’s composition, manufacture and develops a plan for testing the drug on humans. The IND becomes effective if the FDA does not disapprove it within days. Japan’s regulatory system demands the IND Application documents to be prepared in the Common Technical Document (CTD) format. Role in Clinical Trial Approval Process.
The act establishes two time frames for gaining approval : Standard Review and Priority Review. The goal for standard review is to get a drug through the approval process in months. This type of review is applied to a drug that offers little to no improvement over other therapies already on the market. Easily collaborate in teams with the airSlate business automation platform. The IND application should provide high quality preclinical data to justify the testing of the drug in humans.
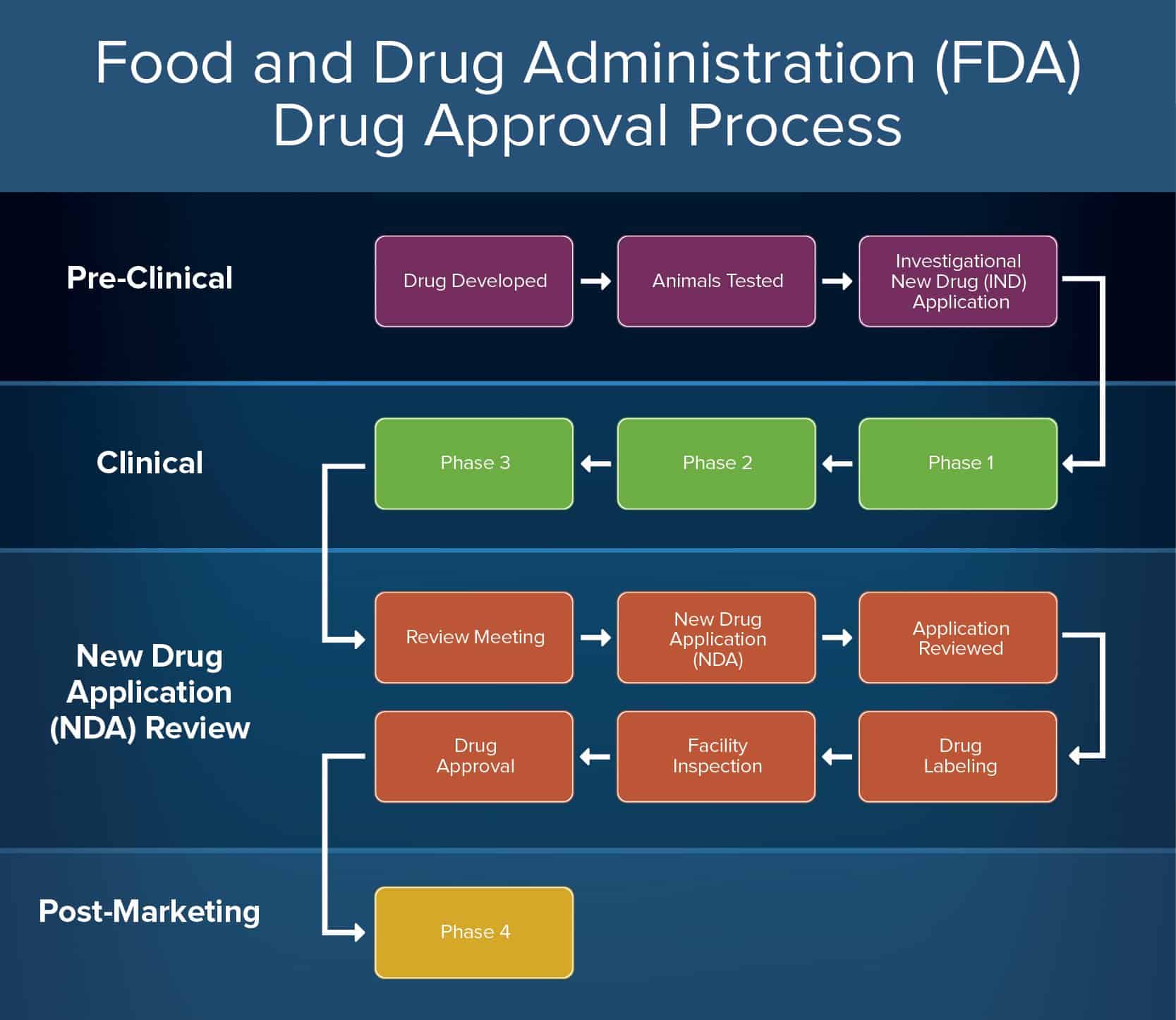
Almost of drugs are subjected to clinical trials, for which IND applications are filed. In the United States, new pharmaceutical products must be approved by the Food and Drug Administration (FDA) as being both safe and effective. Following IND approval , three. Filing an investigational new drug application ( IND ) is a complicated process , and submitting to more than one regulatory body at a time adds a layer or two of complexity.
However, taking the requirements of multiple countries at once can save a great deal of time and avoid rework down the road. That company must seek and receive FDA approval , by way of an investigational new drug ( IND ) application, to test the product with human subjects. The answer to this question depends on your product category. Here are the FDA approval requirements for each category of products i. Overview of pre- IND Process for academics. Request pre- IND mtg FDA will respond with the date days days Pre- IND mtg FDA will send meeting minutes and recommendations days Prepare and submit IND Pre- IND materials due weeks prior.
Pre- IND Request (see handout) Cover Letter (formal, see example) Product Name.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.