The three most commonly occurring scenarios when clinical investigations may be exempted from the IND application requirements refer to certain limited situations of clinical investigations with. IND requirements are codified in Title of the Code of Federal Regulations, Part 3(CFR 312). From a regulatory standpoint, an IND acts as a technical exemption from certain Federal restrictions regarding the transportation and distribution of drugs across state lines.

Exemptions involve a certain type of property , or the property of a certain kind of taxpayer , which is not taxable. Application for exemption must be filed before April of the assessment year with the county assessor. Current Federal law requires that a drug be the subject of an approved marketing application before it is transported or distributed across state lines. Because a sponsor will probably want to ship the investigational drug to clinical investigators in many states, it must seek an exemption from that legal requirement.
The IND is the means through which the sponsor technically obtains this exemption from the FDA. When a product is identified as a viable candidate for further development, the sponsor then focuses on collecting the data and information necessary to establish that the product will not expose humans to unreasonable risks when used in limite early-stage clinical studies. See full list on fda. The following resources include the legal requirements of an IND application, assistance from CDER to help you meet those requirements, and internal IND review principles, policies and procedures. The review divisions are organized generally along therapeutic class and can each be contacted using the designated Pre-IND Consultation List (PDF - 19KB).
They also establish policies intended to achieve. To learn more about property tax exemptions , click HERE. County auditors are the best point of contact for questions regarding deductions and eligibility.
Complete the following checklist to determine if the use of a lawfully marketed drug in your clinical research study meets criteria for IND exemption. All fraternal benefit society benefits are 1 exempt. Every state has its own set of property exemptions. And some states also allow you choose between their exemptions and a set of federal bankruptcy exemptions.
If the drug is not legally markete the study is not eligible for IND exemption and would require an IND. Exemptions (2) through (5) are commonly referred to as “Industrial Exemptions. This article is not offered in support or opposition of Industrial Exemptions, but is offered as background on why exemptions are thought to exist and how they are applied within Florida. The exact legal code can be viewed below. If vaccines go against your family’s sincerely held personal beliefs, click on the appropriate link below to download an exemption letter which you can submit to your school’s nurse or other appropriate person each year.
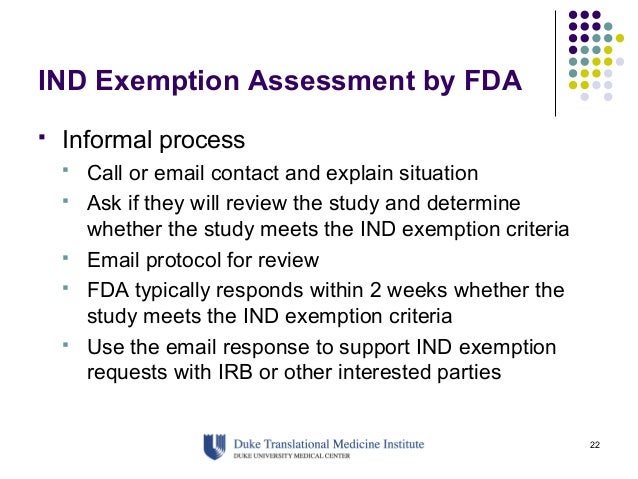
When a trial originates within a research site, an investigator begins what is known as an investigator-initiated trial (IIT). When conducting an IIT, oftentimes, an investigational new drug ( IND ) or investigational device exemption (IDE) application is necessary. An IND is not an application for marketing approval. Food and Drug Administration (FDA) regulations 21CFR312.

Instea it is the way a sponsor gets an exemption to the federal law. Unless a study meets one of the exemptions below, it is subject to IND regulations. Exemption for Clinical Investigations involving a Lawfully Marketed Drug(s) 21CFR312. Changes to the lawfully marketed drug product that do not increase the risks over the risk presented by use of the product are allowed.
Answer each question below. If any question is answered “yes”, an IND application must be submitted to the FDA. If the answer to All questions are “no”, then the study may meet the criteria for an exemption from an IND.

For Research Studies Involving the use of an FDA approved Drug: Does the study involve a different route of administration of the marketed drug than already FDA-approved that significantly increases the risk (or decreases the acceptability of the risks) to study subjects? Here in Florida, the Industrial Exemption is only afforded to those who meet the narrow requirements of F. So regardless of whether an engineer working within Florida is licensed or unlicense they are required to be familiar with Florida’s engineering licensure laws. The amount of each exemption under subsection (c) applies until a rule is adopted by the department of financial institutions under section 2. The New Resident Statement on the front of the form MUST be completed.
Indiana does not recognize the federal exemptions.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.