Review of the NDA typically lasts one to two years, bringing total drug development and approval (that is, the IND and NDA stages) to approximately nine years. During the NDA stage, the FDA consults advisory committees made of experts to obtain a broader range of advice on drug safety, effectiveness, and labeling. The below process chart describes the FDA’s review steps of the NDA : Following the review of data submitted in support of safety and efficacy, FDA will carry out bio research monitoring (BIMO) or cGMP inspections prior to approval. Most of the time, the new agreement’s terms will take precedence. Are you allowed to talk about the negotiation process ? Submission Processing 2. Filing Determination and Review Planning 3. Post-Action Feedback The overall goal of the review process is to provide a thorough but efficient mechanism whereby the safety, effectiveness, and quality of a product can be adequately assessed and a regulatory decision can be made in a timely manner.
The amount of time that applications spend between initial submission and a final regulatory decision varies. Current FDA performance goals under the Prescription Drug User Fee Act (PDUFA) stipulate that FDA intends to review and act on of standard NDA and BLA submissions within months of the filing date. The goal for priority review applications is months. This review target is commonly referred to as the PDUFA goal date.
Real-Time Oncology Revi. See full list on nuventra. Because all commercial marketing applications are now submitted electronically, the first hurdle is to pass an automated technical review. If a submission is missing key components or the study data fail to meet technical conformance criteria, then the application will receive a technical rejection. Assuming the application passes this automated review , it will be further processed and disseminated to the appropriate review division.
The regulatory project manager (RPM) within the review division will ensure that the application meets preliminary regulatory and user fee requirements. If these requirements are met, then a review team is formed. From the time a marketing application is submitte FDA has days to perform an initial review.
Day post-submission is designated as the filing decision date. Prior to the filing decision date, each review discipline will make a recommendation about the “file-ability” of the application. Sponsors should note that their turnaround time to address deficiencies in the 74-Day Letter is limited and that they should plan resourcing accordingly.
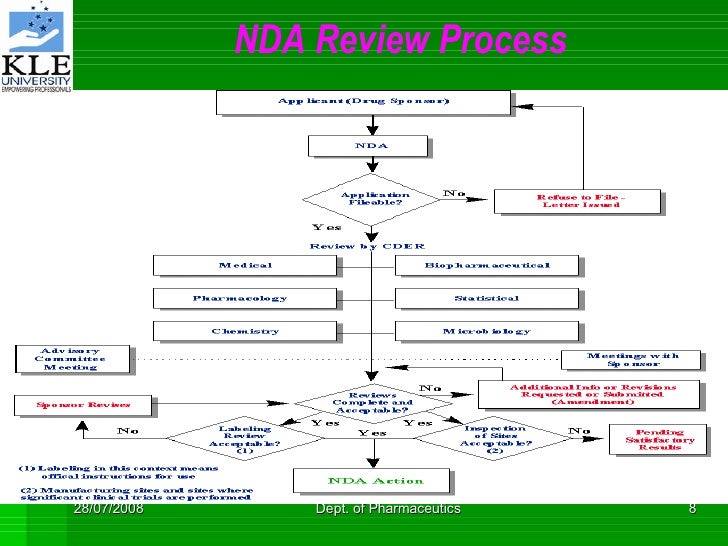
In addition to the filing determination, FDA also. The full review extends from the filing decision to around Month post-submission and to Month for priority applications. This generally involves consults between primary, secondary, and supervisory reviewers, reviewers from other disciplines, and other consultants from the various offices within FDA, as needed. During this phase, FDA performs a detailed review of the application.
Sponsors will likely receive additional information requests from FDA during the full review phase. As part of the review process , there are a number of tasks beyond responding to information requests that require sponsor participation. These include the Safety Update Report, Mid-Cycle Communication, Pre-approval Inspection (PAI), Late-Cycle Meeting, Advisory Committee (AC) Meeting (if required), and Labeling Discussions. Sponsors are required to provide FDA with what is commonly known as a 120-Da. The Action phase involves finalization of the action package.

This includes the Division and Office Director (i.e., signatory) reviews, the final action on the proposed PMC, PMR, REMS, and the official notification of the sponsor. FDA’s determination is conveyed to the sponsor via a formal communication known as an Action Letter. In the event that the signatory authority decides not to approve the application, the RPM drafts a Complete Response (CR) letter, which indicates deficiencies and recommendations for corrective action.
If the application is approve the RPM drafts an Approval Letter. The sponsor may also receive discipline-specific deficiency letters. Following FDA’s decision on approval, the Post-Action phase begins.
This phase presents opportunities for both the FDA and sponsor. The purpose of these communications is to improve the process and to ensure consistency, efficiency, and alignment with PDUFA goals. This meeting is not considered a PDUFA meeting but is intended to help improve processes for future applications. The purpose of this meeting is to ensure that the sponsor understands the deficiencies in the application and the expected responses.
If a sponsor receives a CR letter, then an End-of-Review Conference may be requested by the sponsor, as described in CFR 314. To emphasize, this meeting. The marketing application review process can require considerable sponsor involvement. In order to facilitate efficient review and a timely regulatory decision, sponsors should: 1. Have a firm grasp on the review process 2. Understand sponsor-side post-submission responsibilities 3. Ensure that the original submission is as complete as possible 4. Respond to all information requests in a thoughtful and expeditious manner 5. If you are planning a marketing application submission now or in the future, contact Nuventra to learn how our team of industry veterans can help position your program for success.
Contact Us Additional References 1. New Drug Application and. FDA’s new drug application review process has several strengths that contribute significantly to its effectiveness. Both FDA reviewers and sponsors have confidence in the decisions FDA makes. Our review underscored that FDA’s NDA review process is science-based and comprehensive. What should be included in a NDA?
Despite criticism of the FDA review process , new cancer drugs reach patients sooner in the united states than in Europe. Health Aff (Millwood). Downing NS, Aminawung JA, Shah ND. Regulatory review of novel therapeutics-comparison of three regulatory agencies. Instant Downloa Mail Paper Copy or Hard Copy Delivery, Start and Order Now!
The application must include detailed evidence from a series of clinical. The book provides guidance to medical writers for drafting FDA-submissions in a way more likely to persuade FDA reviewers to grant approval of the. The NDA is the official request for US approval of a drug.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.