How long does the research and approval process last? What is the FDA approval process? Once approved , an applicant may manufacture and market the generic drug product to provide a safe, effective, low cost alternative to the public. Such applications document safety and efficacy of the investigational drug and contain all the information collected during the DDP.
Obtaining approval to market a new drug frequently takes between six months and two years. NDA Review Process 45. American consumers benefit from having access to the safest and most advanced pharmaceutical system in the world. The center ensures that drugs, both brand-name and generic, work correc. See full list on fda.
FDA approval of a drug means that data on the drug’s effects have been reviewed by CDER , and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population. The drug approval process takes place within a structured framework that includes: 1. Analysis of the target condition and available treatments—FDA reviewers analyze the condition or illness for which the drug is intended and evaluate the current treatment landscape, which provide the co. The agency also employs several approaches to encourage the development of certain drugs, especially drugs that may represent the first available treatment for an illness, or ones that have a significant benefit over existing drugs. These approaches, or designations, are meant to address specific needs, and a new drug application may receive more than one designation, if applicable. Each designation helps ensure that therapies for serious conditions are made available to patients as soon as r. CDER Antibacterial Drug Development Task Force 5. Tufts Center for the Study of Drug Development.
Whether the drug is safe and effective in its proposed use(s), and whether the benefits of the drug outweigh the risks. The following drugs have recently been approved by the FDA. Includes newly approved drugs and new indications for drugs already approved. Book Published May. Accelerating Global Registrations.
FDA Review FDA review teams thoroughly examine all of the submitted data related to the drug or device and make a decision to approve or not to approve it. New Drug Approval Process book. The new drug approval is of two phase process - the first phase for clinical trials and second phase for marketing authorization of drug. Firstly, non-clinical studies of a drug are completed to ensure efficacy and safety, and then application for conduct of clinical trials is submitted to the competent authority of the concerned country.

The FDA’s approval process has garnered many criticisms over the years. For one, generic drugs and devices often make it to market simply because the manufacturers can demonstrate they are similar to products that were approved in the past — even if those products have known safety concerns. The show that both aceclofenac and diclofenac in follow-up of days was similar in efficacy and the aceclofenac, new antiinflammatory drug was effective in osteoarthritis treatment, and. Search and discuss any drug with millions of patients.
The FDA must approve new drug compounds before drug companies can market or sell the drug in the U. However, the FDA isn’t responsible for developing drugs , nor does the organization conduct any testing. In reality, the only role the FDA has in the testing of drugs is reviewing data drug sponsors gather and submit. Requirements for permission of new drugs approval.
Post approval changes in biological products: quality, safety and efficacy documents. Preparation of the quality information for drug submission for new drug approval. The FDA approval process can be long, tenuous, and frustrating, especially for patients waiting on new or generic drugs to hit the market.
Although the intention of the process is to ensure patient safety and drug effectiveness, there are some elements that may prove unnecessary and can be expedited under critical circumstances. Drug approval standards in the United States are considered by many to be the most demanding in the world. One of these drugs that are tested in people is approved.
The chance for a new drug to actually make it to market is thus only in 000. The process of drug approval is controlled in most countries by a governmental regulatory agency. Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery.
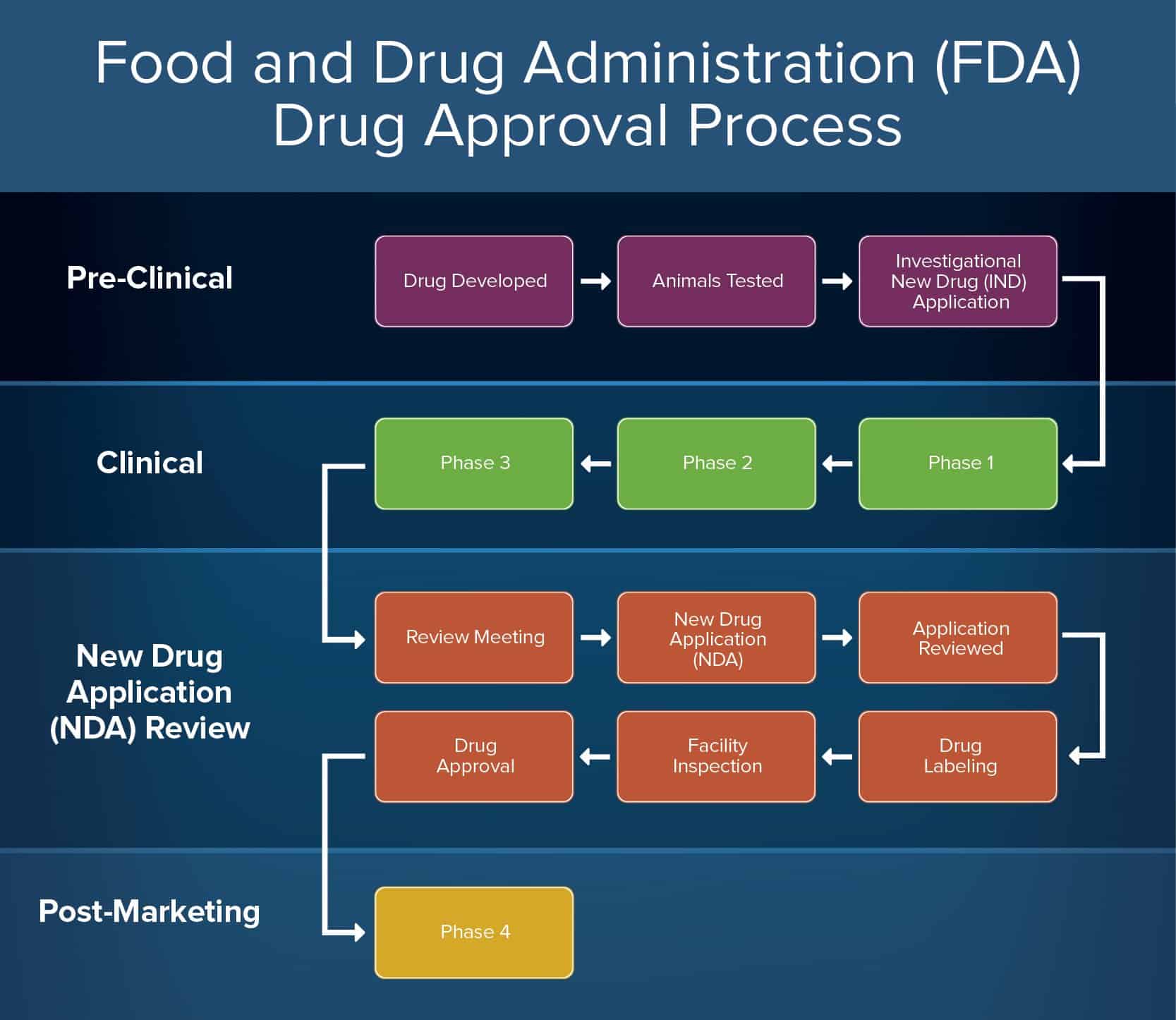
Food and Drug Administration (FDA) governs this process.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.