What are the steps for drug approval? How do you find out if something is FDA approved? What is the FDA approval process like? What do products get approved by the FDA?
American consumers benefit from having access to the safest and most advanced pharmaceutical system in the world. The center ensures that drugs, both brand-name and generic, work correc. See full list on fda. FDA approval of a drug means that data on the drug’s effects have been reviewed by CDER , and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population.
The drug approval process takes place within a structured framework that includes: 1. Analysis of the target condition and available treatments—FDA reviewers analyze the condition or illness for which the drug is intended and evaluate the current treatment landscape, which provide the co. The agency also employs several approaches to encourage the development of certain drugs, especially drugs that may represent the first available treatment for an illness, or ones that have a significant benefit over existing drugs. These approaches, or designations, are meant to address specific needs, and a new drug application may receive more than one designation, if applicable. Each designation helps ensure that therapies for serious conditions are made available to patients as soon as r. Fast Track, Breakthrough Therapy, Accelerated Approval , Priority Review 4. Before a drug company can test an experimental treatment on humans, it must prove the drug is safe.
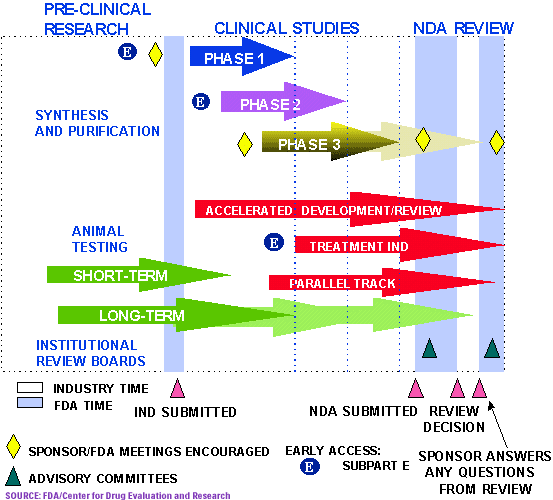
Phase one clinical trial. FDA review teams thoroughly examine all of the submitted data related to the drug or device and make a decision to approve or not to approve it. The FDA reviews information that goes on a drug's professional labeling (information on how to use the drug ). Drug Development Device Development. The FDA inspects the facilities where the drug will be manufactured as part of the. An NDA includes all animal and human.
The Investigational New Drug Process Drug developers , or sponsors, must submit an Investigational New Drug (IND) application to FDA before beginning clinical research. In the IND application ,. The FDA Center for Drug Evaluation and Research (CDER) is the watchdog for potential medications seeking approval for use in the United States. In order for CDER to begin evaluating a drug , pharmaceutical companies must first do extensive testing and document the.
Those are sent in to the CDER, who assigns a team of doctors, chemists, pharmacologists, and other scientists to review the evidence. Generic-drug makers also must gain FDA approval, though they do not need to repeat the clinical trials of. FDA Drug-Approval Process Generic Drugs.
Federal law allows generic-drug companies to work on drugs to gain FDA approval before the patents held by. Here is a complete step -by- step guide for FDA medical device approval process. Medical devices, from ideation to post-launch assessment, are directed in the United States by the U. The NDA must include all data on the animal and human trials, as well as information on how the drug is manufactured. Drug companies continuously analyze thousands of compounds, seeking ones of therapeutic value. The drug and the stop-start study process have been viewed skeptically by some.

The FDA new drug approval process begins with research plans involving basic research, laboratory, and animal testing. This initial stage includes discovery and development of prototypes involving preclinical and clinical studies of new drug materials to be reviewed and approved by an institutional review board (IRB). Food and Drug Administration ( FDA ) is the federal agency responsible for labeling medications and supplements. However, the approval process is different for prescription and for over-the-counter medications. Prescription Drugs The FDA must regulate and approve new prescription drugs before they can be sold to the public.
The sponsor of a new vaccine product follows a multi- step approval process , which typically includes. A FDA Expert Will Answer You Now! Questions Answered Every Seconds.
Search and discuss any drug with millions of patients.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.